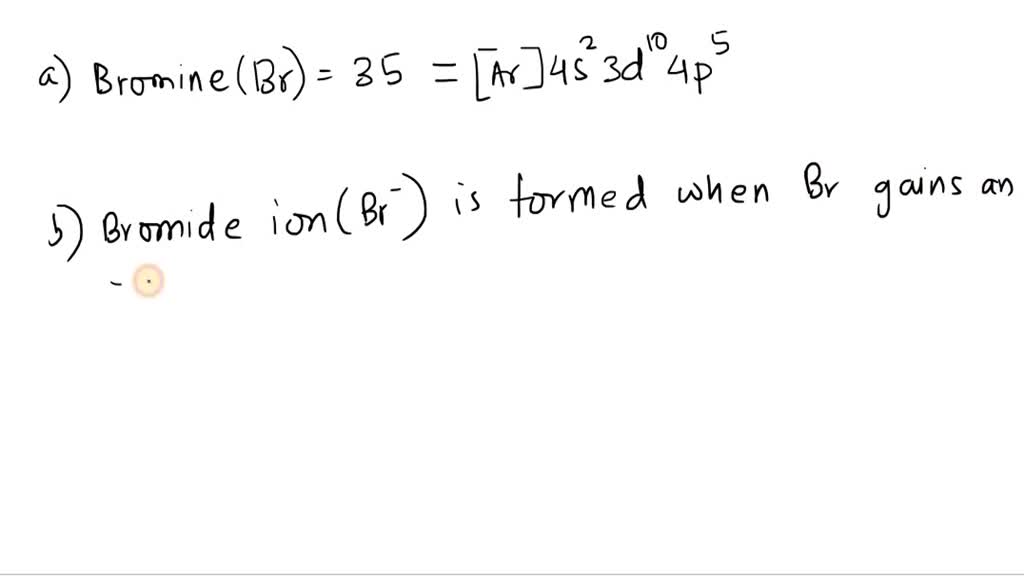Bromine Valence Electrons Core Electrons Unpaired Electrons . For the main group (representative) elements, the valence electrons are the outermost (highest. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: What is the valency of bromine?. valence electrons are the electrons in the outermost shell, or. So in this case, the valency of bromine is 3. One lone pair and three unpaired electrons. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. here, bromine has three unpaired electrons. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons.
from www.numerade.com
based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. For the main group (representative) elements, the valence electrons are the outermost (highest. So in this case, the valency of bromine is 3. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. What is the valency of bromine?. valence electrons are the electrons in the outermost shell, or. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: here, bromine has three unpaired electrons. One lone pair and three unpaired electrons.
SOLVEDWhat is the electron configuration of a bromine atom (You CAN
Bromine Valence Electrons Core Electrons Unpaired Electrons since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. valence electrons are the electrons in the outermost shell, or. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. For the main group (representative) elements, the valence electrons are the outermost (highest. here, bromine has three unpaired electrons. So in this case, the valency of bromine is 3. One lone pair and three unpaired electrons. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: What is the valency of bromine?.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Valence Electrons Core Electrons Unpaired Electrons here, bromine has three unpaired electrons. For the main group (representative) elements, the valence electrons are the outermost (highest. What is the valency of bromine?. valence electrons are the electrons in the outermost shell, or. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. So in this case, the. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Valence Electrons Core Electrons Unpaired Electrons here, bromine has three unpaired electrons. One lone pair and three unpaired electrons. What is the valency of bromine?. For the main group (representative) elements, the valence electrons are the outermost (highest. So in this case, the valency of bromine is 3. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.youtube.com
Know the difference between Valency and Valence electrons in Tamil Bromine Valence Electrons Core Electrons Unpaired Electrons For the main group (representative) elements, the valence electrons are the outermost (highest. So in this case, the valency of bromine is 3. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: What is the valency of bromine?. here, bromine has three unpaired electrons. valence electrons are the electrons in the. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.wou.edu
CH104 Chapter 2 Atoms and The Periodic Table Chemistry Bromine Valence Electrons Core Electrons Unpaired Electrons For the main group (representative) elements, the valence electrons are the outermost (highest. So in this case, the valency of bromine is 3. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: valence electrons are the electrons in the outermost shell, or. What is the valency of bromine?. here, bromine has. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Valence Electrons Core Electrons Unpaired Electrons here, bromine has three unpaired electrons. So in this case, the valency of bromine is 3. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. valence electrons are the electrons in the outermost shell, or. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Valence Electrons Core Electrons Unpaired Electrons For the main group (representative) elements, the valence electrons are the outermost (highest. One lone pair and three unpaired electrons. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: What is the valency of bromine?. So in this case, the valency of bromine is 3. valence electrons are the electrons in the. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.numerade.com
SOLVEDWrite orbital diagrams for the valence electrons and indicate Bromine Valence Electrons Core Electrons Unpaired Electrons valence electrons are the electrons in the outermost shell, or. What is the valency of bromine?. For the main group (representative) elements, the valence electrons are the outermost (highest. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: since the core electron shells correspond to noble gas electron configurations, we can. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Valence Electrons Core Electrons Unpaired Electrons since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. here, bromine has three unpaired electrons. For the main group (representative) elements, the valence electrons are the outermost (highest. One lone pair and three unpaired electrons. valence electrons are the electrons in the outermost shell, or. group 15 elements. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Valence Electrons Core Electrons Unpaired Electrons group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. So in this case, the valency of bromine is 3. One lone pair and three unpaired electrons. What is the valency of bromine?. here, bromine. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Bromine Valence Electrons Core Electrons Unpaired Electrons One lone pair and three unpaired electrons. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. For the main group (representative) elements, the valence electrons are the outermost (highest. here, bromine has three unpaired electrons. What is the valency of bromine?. based on the structures shown above, bromine contains. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Valence Electrons Core Electrons Unpaired Electrons here, bromine has three unpaired electrons. So in this case, the valency of bromine is 3. One lone pair and three unpaired electrons. What is the valency of bromine?. valence electrons are the electrons in the outermost shell, or. For the main group (representative) elements, the valence electrons are the outermost (highest. since the core electron shells. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From valenceelectrons.com
How Many Valence Electrons Does SO2 (Sulfur Dioxide) Have? Bromine Valence Electrons Core Electrons Unpaired Electrons valence electrons are the electrons in the outermost shell, or. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. So in this case, the valency of bromine is 3. here, bromine has three. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Valence Electrons Core Electrons Unpaired Electrons For the main group (representative) elements, the valence electrons are the outermost (highest. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. valence electrons are the electrons in the outermost shell, or. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by.. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From guides.byjusweb.com
Electronic Configuration of Iron Fe element Valency, Applications Bromine Valence Electrons Core Electrons Unpaired Electrons One lone pair and three unpaired electrons. valence electrons are the electrons in the outermost shell, or. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. here, bromine has three unpaired electrons. So in this case, the valency of bromine is 3. For the main group (representative) elements, the. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From megankruwmunoz.blogspot.com
How Many Unpaired Electrons Does P Have MegankruwMunoz Bromine Valence Electrons Core Electrons Unpaired Electrons For the main group (representative) elements, the valence electrons are the outermost (highest. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: What is the valency of bromine?. valence electrons are the electrons in the outermost shell, or. One lone pair and three unpaired electrons. based on the structures shown above,. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Valence Electrons Core Electrons Unpaired Electrons What is the valency of bromine?. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. So in this case, the valency of bromine is 3. One lone pair and three unpaired electrons. valence electrons are the electrons in the outermost shell, or. For the main group (representative) elements, the valence. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From brainly.in
bromine valence electrons, Brainly.in Bromine Valence Electrons Core Electrons Unpaired Electrons valence electrons are the electrons in the outermost shell, or. based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by. What is the valency of bromine?. So in this case, the valency of. Bromine Valence Electrons Core Electrons Unpaired Electrons.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine Valence Electrons Core Electrons Unpaired Electrons So in this case, the valency of bromine is 3. group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. What is the valency of bromine?. here, bromine has three unpaired electrons. since the core electron shells correspond to noble gas electron configurations, we can. Bromine Valence Electrons Core Electrons Unpaired Electrons.